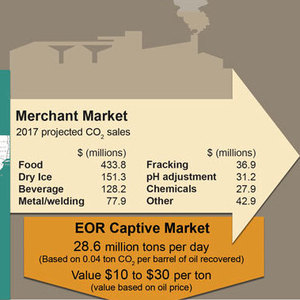
Ethanol Industry Provides Critical CO2 Supply
February 8, 2017
BY Steffen Mueller
Advertisement
Advertisement
Related Stories
Despite its geographical location and forward-thinking approach toward trying new tech, Aemetis Inc. believes its story can be central to the future of biofuels.
SPOTLIGHT | Membrane Technology Is Ushering In a New Era In Ethanol Distillation, Dehydration and Evaporation
Whitefox Technologies is advancing ethanol distillation, dehydration and evaporation (DD&E) by replacing molecular sieves with membrane technology, achieving up to 50% reductions in both energy and water consumption.
U.S. fuel ethanol production fell by nearly 2% the week ending May 2, according to data released by the U.S. Energy Information Administration on May 7. Stocks of fuel ethanol were down 1% and exports were down nearly 9%.
The Andersons Inc. on May 7 released first quarter financial results, reporting a strong three-month period for its renewables business segment on efficient operations, higher yields and favorable ethanol margins.
The U.S. Energy Information Administration maintained its outlook for 2025 and 2026 fuel ethanol production in its latest Short-Term Energy Outlook, released May 6. The outlook for fuel ethanol exports was lowered.





