Consequences of a Lower pH

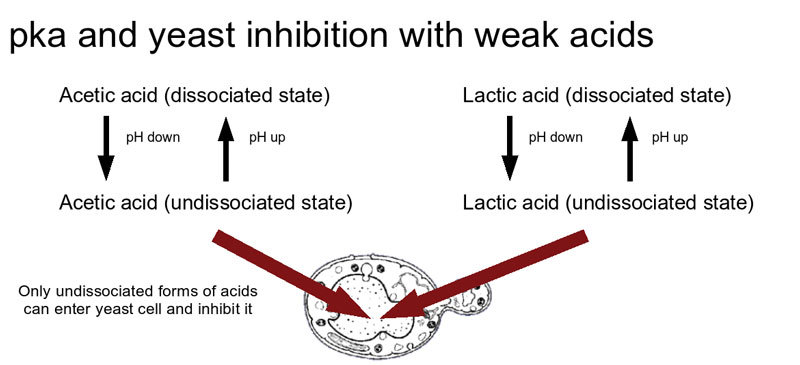
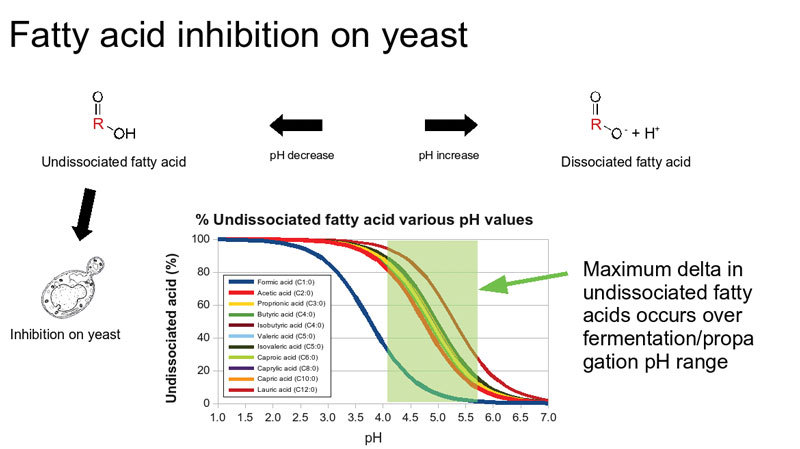

PHOTO: BBI INTERNATIONAL
February 18, 2019
BY Dennis Bayrock
Advertisement
Advertisement
Related Stories
In June, the USGC held its third African Buyers and Sellers Conference in Abidjan, Ivory Coast, bringing together more than 95 attendees from across the continent to meet with U.S. feed grain suppliers and establish trade relationships.
Production and use of renewable ethanol from ePURE members and other EU producers reduced greenhouse-gas emissions by an average of 79% compared to fossil fuels in 2024, according to newly certified data.
U.S. fuel ethanol production was down nearly 3% the week ending June 20, according to data released by the U.S. Energy Information Administration on June 25. Stocks of fuel ethanol were up 1% and exports fell by 33%.
The USDA recently released its Grain Crushings and Co-Products Production report for June, reporting that corn use for fuel ethanol in April was down when compared to the previous month, but up when compared to April of last year.
In June, the U.S. Grains Council signed a renewed MOU with Vietnam’s Ministry of Agriculture and Environment to strengthen bilateral trade of animal feed and biofuels and expand technical support to the Vietnamese agricultural industry.





