Experiment Design Validates Measurement Technology

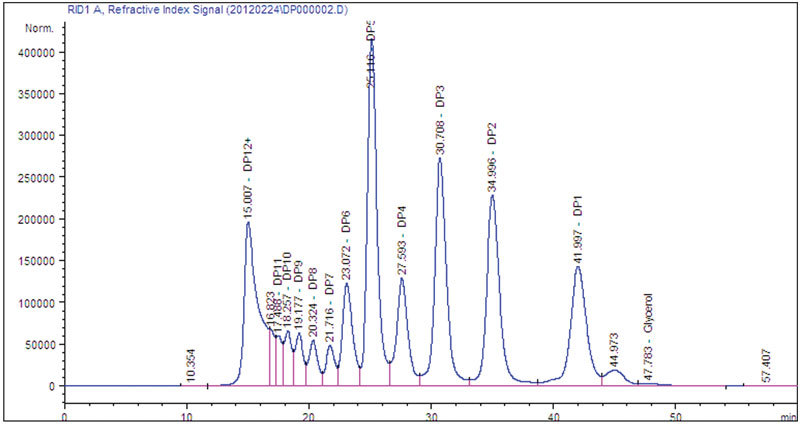
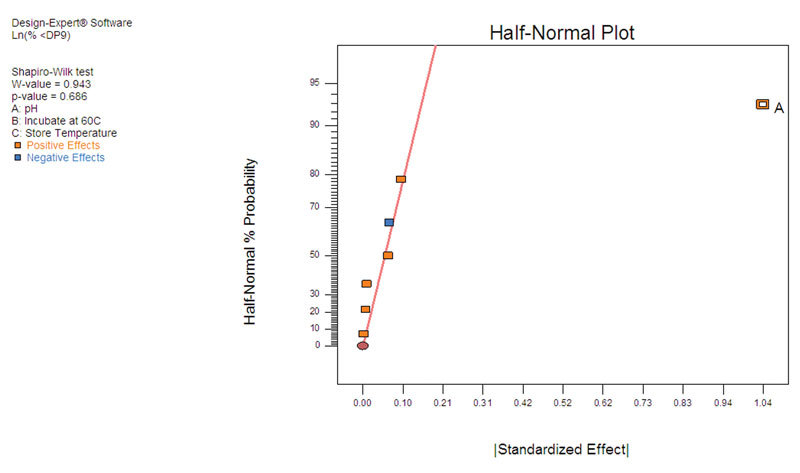
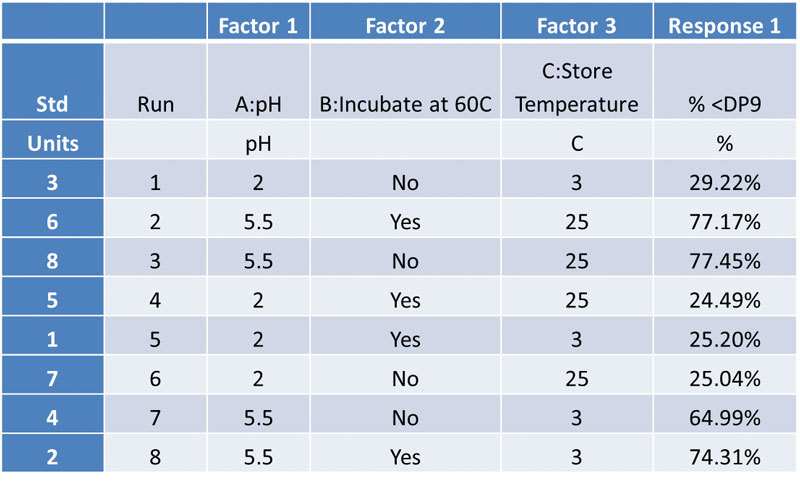

PHOTO: Susanne Retka Schill, BBI International
May 21, 2014
BY Ron Stites
Advertisement
Advertisement
Related Stories
Representatives from the USGC, Growth Energy and the RFA recently conducted a strategic mission to Southeast Asia (SEA), underscoring the U.S. ethanol industry’s support for ethanol policy and technical advancement in Vietnam and the Philippines.
Production and use of renewable ethanol from ePURE members and other EU producers reduced greenhouse-gas emissions by an average of 79% compared to fossil fuels in 2024, according to newly certified data.
Greene Tweed, a global leader in advanced materials and high-performance solutions, has released results from a study confirming the compatibility of its fluorine-based elastomer seals with sustainable aviation fuels (SAF).
The National Corn Growers Association is calling on entrepreneurs, researchers, and startups to reimagine the potential of field corn by entering the fifth round of the Consider Corn Challenge. Applications are due June 30.
Legislation introduced in the California Senate on June 23 aims to cap the price of Low Carbon Fuel Standard credits as part of a larger effort to overhaul the state’s fuel regulations and mitigate rising gas prices.





