Beyond Feed



SOURCE: 2013 DATA FROM THE RENEWABLE FUELS ASSOCIATION
June 10, 2014
BY Susanne Retka Schill
Advertisement
Advertisement
Related Stories
On May 6, the Nebraska Ethanol Board joined Nebraska Gov. Jim Pillen in proclaiming May as Renewable Fuels Month. Nebraska is the country’s second largest ethanol producer, with more than 2 billion gallons of production capacity.
The Trump administration on May 8 announced a new trade deal with the U.K. that the White House said will create a $5 billion export opportunity for U.S. farmers, ranchers and producers, including more than $700 million in ethanol exports.
Reps. Zach Nunn, R-Iowa, and Nikki Budzinski, D-Ill., on May 7 introduced a bill that aims to update USDA’s Section 9003 program to expand access to grants, streamline loan guarantees and provide $100 million in mandatory funding over five years.
Ethanol Producer Magazine has announced the keynote speakers for the 2025 International Fuel Ethanol Workshop & Expo (FEW) being held June 9-11, 2025, at the CHI Health Center in Omaha, Nebraska. The general session will take place June 10.
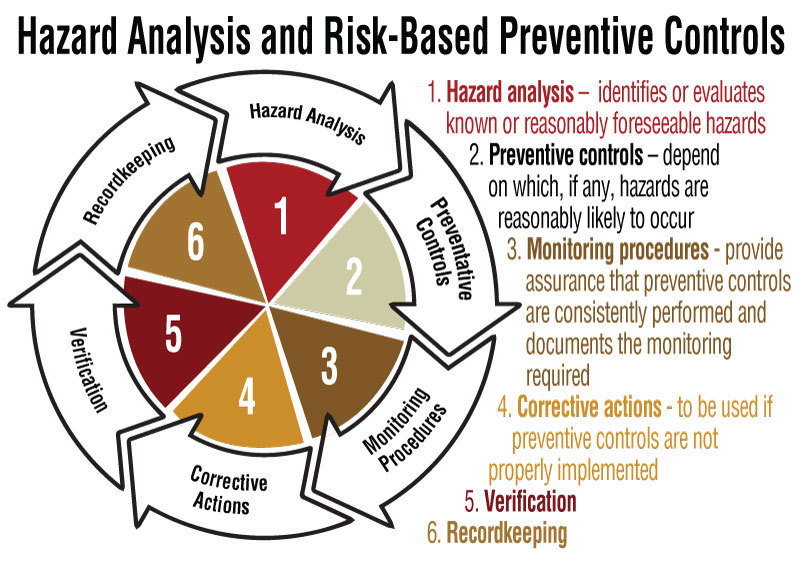
Here is what you need to know about third-party verification.





