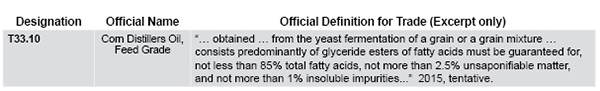
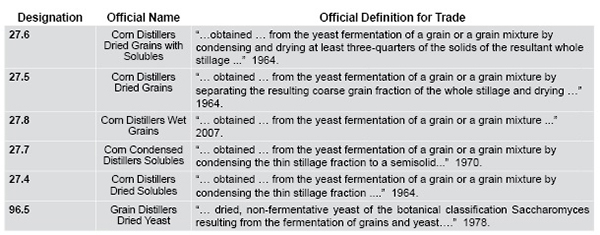
New, Evolving Coproducts Require Regulatory Due Diligence


April 5, 2016
BY Kurt A. Rosentrator
Advertisement
Advertisement
Related Stories
LanzaJet and BioD have launched a feasibility study to develop the first sustainable aviation fuel (SAF) production plant in Colombia. The proposed facility would utilize LanzaJet’s alcohol-to-jet (ATJ) technology.
Behind the scenes with IFF’s biofuel team and its work to bring new yeasts, yeast blends, AI and next-gen research to the ethanol industry.
Model E delivers dependable performance wherever ethanol analysis is needed.
Veolia’s novel corrosion inhibitor makes it easy on ethanol producers by ensuring simple and reliable testing, and performance that significantly cuts traditional dosage rates.
On May 6, the Nebraska Ethanol Board joined Nebraska Gov. Jim Pillen in proclaiming May as Renewable Fuels Month. Nebraska is the country’s second largest ethanol producer, with more than 2 billion gallons of production capacity.



